Cultivation of CHO- and HEK cells
Animal and human cells such as CHO- and HEK cells are relatively large and therefore easily "sink" to the bottom of the cultivation vessel if the degree of turbulence inside the culture is insufficient. This leads to a requirement for quite vigorous shaking conditions, though one should also take into account that the shear forces should not be too high either. Thirdly, the degree of splashing should be minimized.
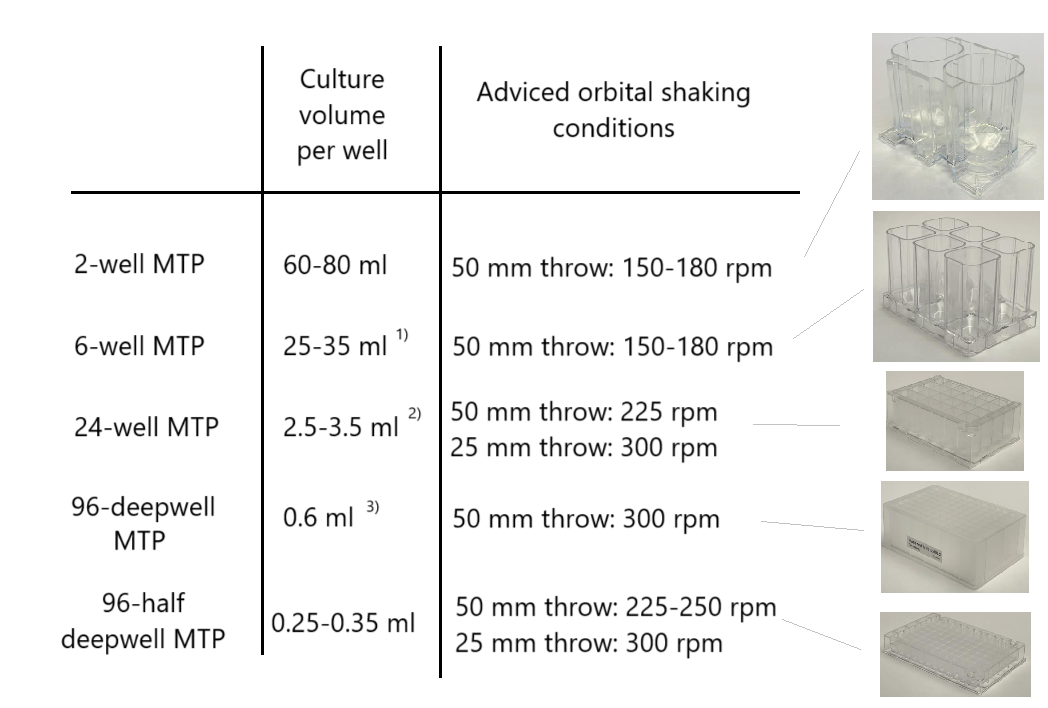
Individual animal-cell lines differ in their tendency to settle, and in their susceptibility to shear forces. Therefore, the adviced shaking conditions and culture volumes for the various types of microplates in the table below (based on feedback from users) are to be used as a starting point for further optimization. The parameter that is most easy to vary in one optimization experiment is the culture volume; use 4 or 6 different culture volumes (e.g. 2, 2.5, 3, 3.5, 4, and 4.5 ml in a 24-square deepwell plates), and quantify titers and CFUs in the course of the experiment. If relatively large culture volumes give the best results, this may be an indication i) that your particular cell line has little tendency to settle, and/or ii) that this particular cell line thrives best under mild-turbulence conditions (and so me be relatively sucesptible to shear forces).
In general, we advice to use shakers with a large shaking diameter (25mm or - preferably 50 mm). This is the easiest way to achieve the desired turbulence (prevent "dead zones"), without imposing too high shear forces, and to avoid splashing.
With regard to the differences in hydrodynamics between the various microplates, the following considerations: with small wells (as in the 96-well plates), the criterium is quite strict: the culture should be mixed "down-to-the-bottom" (as illustraded in the videos on: Enzyscreen - Hydrodynamics inside wells of a 96-square deepwell microplate ; if not, settling of cells is unavoidable. With larger cultures (as in the 2 and 6-well MTPs; 24-well MTPs take an intermediate position here), the need for mixing "down-to-the-bottom" is less strict, because the "swirling speed" (the speed with which the culture travels along the well walls) of the culture is higher: the "swirling speed" is proportional to the well width. This higher "swirling speed" in larger well causes extra turbulence and sort of "eddies", also at the bottom of the wells. Therefore, the chance of settling of cells is diminished in larger wells, even if not mixed "down-to-the-bottom"
Further:
- if properly mixed, oxygen-transfer rates are in practice always sufficient to meet the oxygen demand of animal cell cultures: this is no issue to take into account when choosing the culture conditions (in contrast to bacterial cultures cultures where it is exactly the other way around)
- since the oxygen demand of animal cells is generally quite low, the headspace refreshment rate does only need to be in the order of 0.3 VVM. Therefore, we advice to use our "low-evaporation" sandwich covers ( Enzyscreen - Functions & use of sandwich covers ); in that case the evaporation over a period of 10-14 days is not more than a few percent of the original culture volume (if the shaker cabinet is humidified to 75-85%)
- if feeding of the cultures is required (e.g. every 3 or 4 days, using a multipipette, in a laminar flow cabinet), it is generally advisable to use deepwell microtiter plates, and not too large culture volumes: then the top of the microplate is sufficiently high above the culture itself, thus greatly reducing the chance of cross-contamination when the cover is taken off, and put on again after the feeding procedure
- when cultures need to be fed, some users deliberately use "normal" sandwich covers (bigger diffussion holes) so that the evaporation is higher; this allows a larger (and so more accurate) feeding volume (with a more dilute medium of course), while keeping the culture volume more-or-less constant throughout the experiment.
Contact us
The best way to contact us is to use the form below. However, you may also contact Wouter Duetz directly by e-mail. This e-mail address can be found on the "about us" page.
Optionally you may provide us with additional details about your project; culture type & duration, throughput and desired culture volumes.
We are happy to assist you. We generally reply within a single business day.
